Now that we have gone over how autophagy works, the signaling pathways involved, and how it can be influenced through different factors such as fasting, I will now elaborate further on these topics starting with the concept of mitophagy.
Mitophagy
Mitophagy is the autophagic process of clearing up malfunctional or non-useful mitochondria. The mitochondria are the powerhouses of cells which, through cellular respiration, provide chemical energy (in the form of ATP) to our bodies. In regards to longevity, the mitochondria are an extremely important point of focus due to the fact that they are key players within the processes of aging and are correlated to a large sum of age-related diseases. This is why autophagy, or more importantly, mitophagy, is extremely crucial to sustain a healthy lifespan. There are some theories that claim that oxidative damage accumulated over time due to reactive oxygenated species (ROS) primarily stems from the decline in function of cellular respiration within the mitochondria. Research from scientists such as Varghese and associates, suggest that the consumption of certain natural products such as resveratrol, hydroxytyrosol, and spermidine, amongst others, can sustain or even enhance the action of mitophagy.
Spermidine, Hydroxytyrosol, & Resveratrol
Spermidine is a natural calorie restriction mimetic, found in soy products, whole grains, legumes, and mushrooms, amongst others, which stimulates autophagy. In the previous article, I briefly mentioned how rapamycin, spermidine and metformin act on the autophagic pathways in different fashions. Spermidine has also been found to regulate markers involved in mitophagy.
Hydroxytyrosol is the predominant polyphenol contained in olives and are linked to a vast array of benefits in regards to age-related diseases as well as is linked to decreased oxidative stress, cell death and mitochondrial dysfunction according to De Pablos and associates. It has been found that hydroxytyrosol, in fact, aids the biological functions as well as deters the onset of age-related diseases due to its enhancing effects on autophagy, especially that of mitophagy.
Resveratrol is a naturally occurring polyphenol, that possesses a high antioxidant and anticancer potential. It is found naturally in many foods, one of which is quite popular, is that of red wine and/or grapes. As explained in my article on sirtuins, resveratrol is an activator of SIRT1. Sirtuins govern many biological functions and pathways. It should come as no surprise that sirtuins also have a modulating role on autophagy, which I will return to later in this article. It has also been suggested that fasting/calorie restriction, and resveratrol stimulate sirtuin activity. You can now see a trend that our longevity contenders have tendencies in working together for the common outcome of healthy longevity through similar pathways.
Calorie Restriction/Fasting
As stated in the one of the Healthy Aging blog posts on the Vitoli blog by Eric Simard, PhD in biology, calorie restriction enables:
- Improved glucose management
- Reduced inflammation and body weight
- Lower triglycerides and bad blood cholesterol
- An increase of good cholesterol
- Decreased levels of growth hormones
- Reduced risk of cancer
- Preserved functions of stem cells
- Improved metabolism of mitochondria
- Reduced loss of muscle mass
- Preserved structure and cognitive function
You can see here, that calorie restriction not only aids autophagy function, but is considered to improve metabolism within the mitochondria as well. Keep in mind however, that calorie restriction is associated to these many benefits, and upon including natural products along with calorie restriction mimetics, one would create a well-rounded foundation for protecting the action of autophagy; specifically, mitophagy, thereby delaying or even inhibiting the onset of age-related diseases. As depicted from the image below, autophagy is involved with many longevity mechanisms, and once again, shows its dominance as a key player within this field.
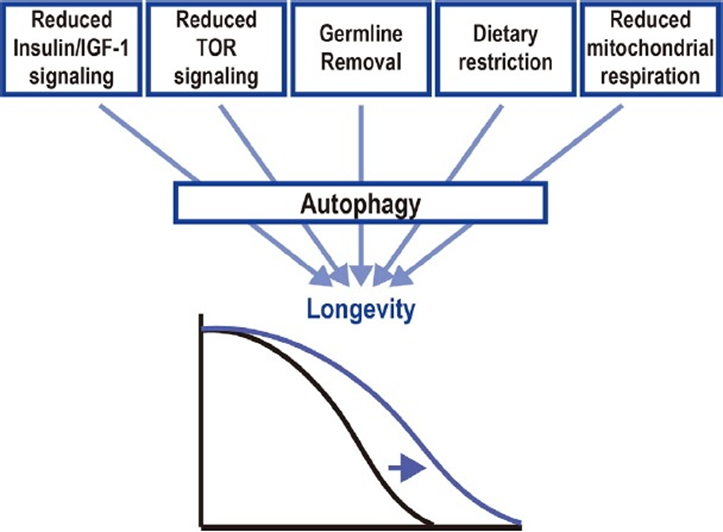
“Autophagy is a convergent mechanism of multiple longevity paradigms” (Nakamura et al., 2018)
Sirtuins
Not only do sirtuins take the lead on multiple biological processes, but, as previously mentioned, sirtuins play a role in the processes of autophagy as well. In a 2019 review article, Dr. Lee states that “overall, mitochondrial sirtuins regulate mitochondrial protein networks, orchestrate mitochondrial function, and allow cells to adapt to metabolic stresses”. Sirtuins once again prove much potential as being an area of focus for finding therapeutics which could sustain overall health for longer periods of time.
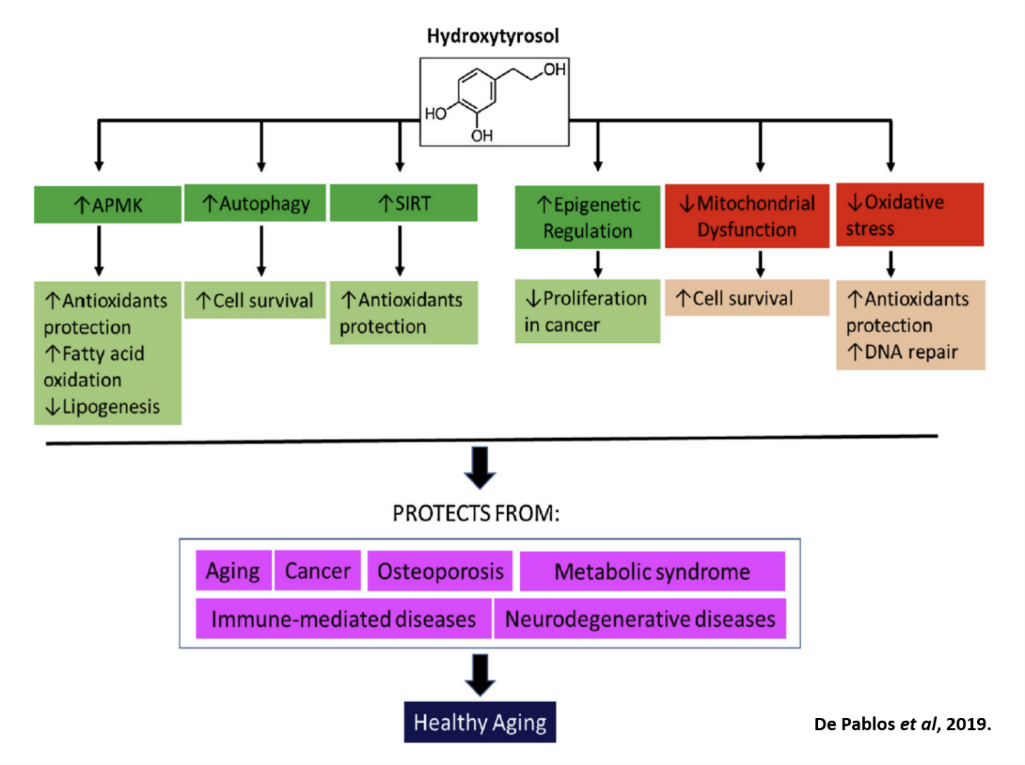
Perhaps it would be at this point where the image below from the article on the Vitoli blog, Olive Polyphenols Protect Against Aging (Article 1 of 2), would make more sense, concerning how hydroxytyrosol affects the action of AMPK, autophagy, sirtuins, and the decrease of mitochondrial dysfunction. You can see here, how many different topics on longevity have now come full circle. Although the image illustrated is a flow chart of how one facet of health can elicit certain biological outcomes, we know very well, that these facets also affect one another in a cyclic as well as diagonal fashion. You could only imagine what the diagram would look like, if natural products and calorie restriction were introduced. It would be quite messy, but nonetheless very complete in demonstrating how fascinating our bodies function at the microcosmic scale.
Final Words
Overall, there are many intricacies and concepts still being researched, but the potential within the field of disease and longevity is vast considering the implications of autophagy on longevity. With key modulators such as sirtuins, and key influencers such as fasting, resveratrol and hydroxytyrosol, the foundation of healthy aging could be well established. It is important to understand the fundamentals of longevity in order to incorporate a preventive lifestyle for healthy aging, along with being mindful of quality products. Now that you are more knowledgeable of these different topics and themes of longevity, diseases and their onset; stay tuned, as my next article will highlight the hallmarks of longevity and aging!
I hope you enjoyed this article and I look forward to writing more on other relevant health topics! Feel free to message us with any comments or enquiries you may have.
Info@vitoli.com
Other suggested articles:
- Olive Polyphenols and Vitoli Products – Article 1: Primary Aging
- Olive Polyphenols and Vitoli Products – Article 2: The Benefits
- A Treatment for Fibromyalgia!?
- Longevity? Prevention? Disease? – Who Cares!
References:
- Bagherniya M, Butler AE, Barreto GE, Sahebkar A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res Rev. 2018;47:183-197. doi:10.1016/j.arr.2018.08.004
- Morselli E, Maiuri MC, Markaki M, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1(1):e10. Doi:10.1038/cddis.2009.8
- Varghese N, Werner S, Grimm A, Eckert A. Dietary Mitophagy Enhancer: A Strategy for Healthy Brain Aging? Antioxidants (Basel). 2020 Sep 29;9(10):E932. doi: 10.3390/antiox9100932. PMID: 33003315.
- Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6(6):702-710. doi:10.4161/auto.6.6.12376
- Antunes F, Erustes AG, Costa AJ, et al. Autophagy and intermittent fasting: the connection for cancer therapy?. Clinics (Sao Paulo). 2018;73(suppl 1):e814s. Published 2018 Dec 10. doi:10.6061/clinics/2018/e814s
- Cuervo, A., Wong, E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res 24, 92–104 (2013). https://doi.org/10.1038/cr.2013.153
- Bejarano E, Cuervo AM. Chaperone-mediated autophagy. Proc Am Thorac Soc. 2010;7(1):29-39. doi:10.1513/pats.200909-102JS
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3-12. doi:10.1002/path.2697
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460-473. doi:10.1089/ars.2013.5371
- Schuck, Sebastian. Microautophagy – distinct molecular mechanisms handle cargoes of many sizes. Journal of Cell Science. Published 9 September 2020; 133: jcs246322 doi: 10.1242/jcs.246322
- Ju Huang & Daniel J. Klionsky (2007) Autophagy and Human Disease, Cell Cycle, 6:15, 1837-1849, DOI: 10.4161/cc.6.15.4511
- Nakamura S, Yoshimori T. Autophagy and Longevity. Mol Cells. 2018;41(1):65-72. doi:10.14348/molcells.2018.2333
- Hannan MA, Rahman MA, Rahman MS, et al. Intermittent fasting, a possible priming tool for host defense against SARS-CoV-2 infection: Crosstalk among calorie restriction, autophagy and immune response. Immunol Lett. 2020;226:38-45. doi:10.1016/j.imlet.2020.07.001
- Chung KW, Chung HY. The Effects of Calorie Restriction on Autophagy: Role on Aging Intervention. Nutrients. 2019;11(12):2923. Published 2019 Dec 2. doi:10.3390/nu11122923
- Blagosklonny MV. From causes of aging to death from COVID-19. Aging (Albany NY). 2020 Jun 12;12(11):10004-10021. doi: 10.18632/aging.103493. Epub 2020 Jun 12. PMID: 32534452; PMCID: PMC7346074.
- Tian Y, Song W, Li D, Cai L, Zhao Y. Resveratrol As A Natural Regulator Of Autophagy For Prevention And Treatment Of Cancer. Onco Targets Ther. 2019;12:8601-8609. Published 2019 Oct 17. doi:10.2147/OTT.S213043
- de Pablos et al, 2019. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immunemediated and neurodegenerative diseases. Pharmacological Research 143 (2019) 58–72.
- Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell. 2016;61(5):654-666. doi:10.1016/j.molcel.2016.01.028
- Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013 Dec;228(12):2262-70. doi: 10.1002/jcp.24399. PMID: 23696314.
- Lee IH, Yun J, Finkel T. The emerging links between sirtuins and autophagy. Methods Mol Biol. 2013;1077:259-271. doi:10.1007/978-1-62703-637-5_17
- Lee, I.H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Exp Mol Med 51, 1–11 (2019). https://doi.org/10.1038/s12276-019-0302-7