Table of Contents
The body is a complex system that is truly remarkable. As I previously explained in the article, COVID-19 and the Concept of Anti-Aging as a Prevention, the body has different forms of maintenance programs to ensure that the intricate parts of the whole are functional. It is quite philosophical to think of our bodies as a complex automated system, as we carry out our day to day lives without having any idea of what is really going on, on a micro-scale or even macro, in the case of a heartbeat!
In order to keep such a complex system running at optimal conditions, when malfunctions arise, your maintenance programs come into play. I will use this opportunity to delve deeper into the world of autophagy and its role in our biological systems, along with its role in regards to disease and longevity. Since there is so much to discuss pertaining to this specific topic, I will separate it into multiple segments. I know that these themes can often get a little heavy, especially if many of these terms are new, hence the need for the well-deserved coffee break! In my next articles will explore the implication of natural products and healthy lifestyles in regards to autophagy.
A Biological Recycling System
Autophagy is a “clean-up” system (what scientists refer to as having a “housekeeping role”) that will swoop in and take care of problematic issues when something malfunctions or is under stress, before any major damage can come into effect. Autophagy is very important in human health because these recycling mechanisms can make functional cells from internal mechanisms which have been damaged or even from released debris that interferes with the proper functioning of cells, as is the case with Alzheimer’s disease. It is an extremely crucial process which occurs under certain circumstances, which include:
- Development stages
- A response to nutritional stress
- When there is misfolding or aggregated proteins
- To clear damaged organelles (mitochondria, endoplasmic reticulum and peroxisomes)
- To eliminate intracellular pathogens
- Low oxygen levels
Efficiency Above All
Your body wants to be as efficient as possible, and when it comes to its resources, it wants to make the best out of any given situation. In order to survive, this is essential! What is important to highlight here, is that when the processes of autophagy come into play, it not only responds to the above conditions, but in achieving the above tasks due to a malfunction or biological stress, it will therefore:
- Promote cellular senescence
- Enable cell surface antigen presentation (which is a form of cellular communication (i.e. cellular signaling), so that the body understands what to do next, such as trigger an immune response etc.)
- Protect against genome instability
- Prevent necrosis (premature death of living cells or body tissues)
- Mobilize energy reserves
- Suppress tumor formation
- Recycle damaged organelles in conditions where there is: Endoplasmic reticulum stress, Hypoxia (tissue deprivation from adequate supplies of oxygen), A lack of nutrients, An accumulation of misfolded proteins, or DNA damage.
When life gives you lemons, autophagy definitely knows how to make lemonade! Since it has such an essential role in humans (and organisms in general), this too is regulated and categorized into three types; all of which promote the degradation of malfunctional/invading cytosolic components via the lysosome. These three types are:
- Microautophagy,
- Macroautophagy, and
- Chaperone-mediated autophagy
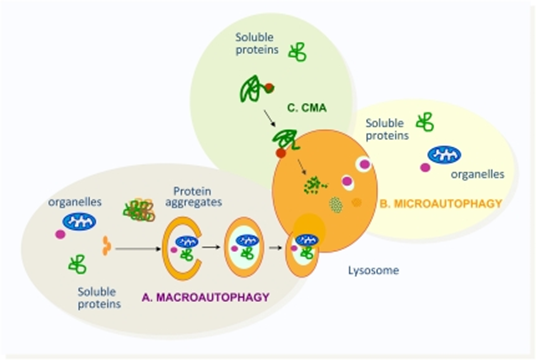
“Types of autophagy described in mammalian cells” (Bejarano et al., 2010)
1. Microautophagy:
As illustrated in the image above on the right, by Bajarano and associates, microautophagy is the degradation process on a micro-scale. Microautophagy is responsible for the degradation of soluble proteins and organelles. These components are directed towards the lysosome and are directly taken into the lysosome through a form of invagination from the lysosomal membrane. The exact mechanism behind microautophagy is still being investigated by researchers, but it is thought to be the main type of autophagy responsible for the turnover of organelles and proteins in all cells. In humans, when there is an amino acid deprivation, cytosolic proteins are degraded via this type of autophagy. It has the ability to work with selective and non-selective mechanisms which entails that some of these processes requires specific signaling and choreographed steps, whereas some do not.
2. Macroautophagy:
Macroautophagy is one of the most studied of all types of autophagy and is linked to many diseases in humans (something which I will return to later in this article as well as articles to come). It usually delivers components such as damaged organelles or outlived proteins, very much like microautophagy, but incorporates protein aggregates as well within its very different mechanism of transport for bulk degradation. Very much like microautophagy, it also works under selective and non-selective mechanisms. Macroautophagy creates a phagophore which once complete, is called the autophagosome, which surrounds the desired component (what scientists may refer to as cytoplasmic/autophagic cargo) that will be sent to the lysosome for degradation. We could draw a parallel with the trucks which recover our recyclable materials. These are transport vehicles that bring damaged materials to the recovery apparatus (lysosomes). This autophagosome is a double membrane-bound vesicle which fuses with the membrane of the lysosome in order to expel its components for degradation (as depicted in the illustration below).
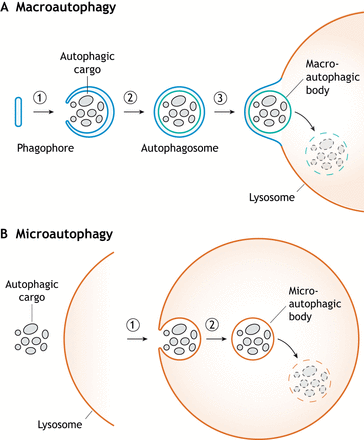
“Membrane transformations during macroautophagy and microautophagy” (Schuck, 2020)
3. Chaperone-Mediated Autophagy (CMA):
Chaperone-mediated autophagy (CMA) works very differently than the other two types. Here (much like you would normally define it as someone who would tag along to oversee something/supervise), a chaperone complex is required in order to target soluble proteins to be brought to the lysosome for degradation. It works only under selective mechanisms which is much more complex in that the chaperone proteins (such as heat shock proteins) is recognized by a lysosomal receptor (lysosomal-associated membrane protein), which work together to, in many cases, unfold the proteins to deliver its cargo within the lysosome. It’s as if a used car dealership worker spotted old cars that can be destroyed to salvage for metal, and gave them a “to destroy” sign.
CMA is solely dedicated to the degradation of soluble proteins through the selective process of recognition from the protein’s amino acid sequences, and is quite the lengthy process whereby it must undergo many requirements into order for the cargo to completely enter within the lysosome. What makes CMA so interesting is that this process is so specific, that it can target particular proteins without disturbing the surrounding proteins in an efficient and effective manner. To continue with the same example, CMA is capable of selecting the cars that no longer work. The energetic, signaling, and stressor requirements along with many of the specific details regarding the processes of CMA are still being researched to this day.
Autophagy & Longevity
It is already known that autophagy processes are more efficient in organisms with a longer lifespan. Throughout the lifespan, the processes of autophagy have tendencies of decreasing in function. Other factors such as poor diet also have a tendency of decreasing their function, which in turn, could promote the onset on many diseases. You can see here, that it’s very much like the concept of the chicken and the egg, where the decrease in the function of autophagy can induce disease, whereas, the function of autophagy tends to decrease with age, therefore inducing the onset of age-related diseases. I will elaborate further on the subject on longevity in, more or less, all of the segments on autophagy, so I recommend keeping an eye out on the Vitoli blog for the newest articles, along with the many other subjects regarding other facets of health.
Autophagy & Disease
Autophagy plays many major roles within our systems, ranging from tumor suppression, to promoting cellular survival, cell death and cellular recycling. What it comes down to, is the fact that autophagy is a cellular adaptation system to various types of stress. Stress is defined here as a factor which changes homeostatic conditions, whereby the body must adapt in order achieve balance once again. In my next article, I will specifically discuss the topic of a particular stress we all know and possibly factor into our daily lives; the concept of fasting.
As stated by Glick and associates, “In addition to elimination of intracellular aggregates and damaged organelles, autophagy promotes cellular senescence and cell surface antigen presentation, protects against genome instability and prevents necrosis, giving it a key role in preventing diseases such as cancer, neurodegeneration, cardiomyopathy, diabetes, liver disease, autoimmune diseases and infections.” Some of the many diseases associated to the mechanisms and/or the dysfunction of autophagy include:
- Parkinson’s disease,
- Huntington Disease,
- Amyotrophic Lateral Sclerosis (ALS),
- Alzheimer’s disease,
- Frontotemporal Lobar Degeneration (FTLD),
- Danon disease,
- Crohn’s disease,
- Breast, ovarian, colon, and prostate cancer,
- Batten disease,
- Paget’s disease,
- Etc.
“However, the significance of defects in autophagy for disease and ageing is apparent from growing evidence linking mutation or loss of function of key autophagy genes in cancer, neuropathies, heart disease, auto-immune disease and other conditions. From the perspective of a cancer biologist, it remains controversial whether autophagy is tumour suppressive (through cell cycle arrest, promoting genome and organelle integrity, or through inhibition of necrosis and inflammation) or oncogenic (by promoting cell survival in the face of spontaneous or induced nutrient stress). In other diseases, such as neuropathies (Huntington’s, Alzheimer’s and Parkinson’s diseases) and ischaemic heart disease, autophagy is more widely accepted as beneficial given its role in eliminating ‘toxic assets’ and promoting cell viability. Thus, autophagy has emerged as a new and potent modulator of disease progression that is both scientifically intriguing and clinically relevant.”
(Glick et al., 2010)
Although autophagy has a vast capability in maintaining health and longevity, autophagy also has its negative implications on health as well. Certain pathogens (bacteria and viruses) can sometimes manipulate these amazing mechanisms to their own advantage, and cancers sometimes utilize the cytoprotective mechanisms which henceforth enables the cancer resistant to many treatments. This is why the full mechanisms as well as their corresponding triggers/signaling is still investigated by researchers. There is much potential for these systems to be targeted through treatments whereby it could disable pathogen/cancer manipulation of these mechanisms as well as target its onset in order to avoid disease altogether.
I hope you enjoyed this article and I look forward to writing more on other relevant health topics! Feel free to message us with any comments or enquiries you may have.
Info@vitoli.com
Other suggested topics:
- Fasting, Article 1: The Biological Basics of Reflection
- Natural Products and Sirtuins; How To Prevent Disease
- COVID-19 and the Concept of Anti-Aging as a Prevention?
- Immune System 2 of 2: 6 Supplements That Work
References:
- Bagherniya M, Butler AE, Barreto GE, Sahebkar A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res Rev. 2018;47:183-197. doi:10.1016/j.arr.2018.08.004
- Morselli E, Maiuri MC, Markaki M, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1(1):e10. Doi:10.1038/cddis.2009.8
- Varghese N, Werner S, Grimm A, Eckert A. Dietary Mitophagy Enhancer: A Strategy for Healthy Brain Aging? Antioxidants (Basel). 2020 Sep 29;9(10):E932. doi: 10.3390/antiox9100932. PMID: 33003315.
- Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6(6):702-710. doi:10.4161/auto.6.6.12376
- Antunes F, Erustes AG, Costa AJ, et al. Autophagy and intermittent fasting: the connection for cancer therapy?. Clinics (Sao Paulo). 2018;73(suppl 1):e814s. Published 2018 Dec 10. doi:10.6061/clinics/2018/e814s
- Cuervo, A., Wong, E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res 24, 92–104 (2013). https://doi.org/10.1038/cr.2013.153
- Bejarano E, Cuervo AM. Chaperone-mediated autophagy. Proc Am Thorac Soc. 2010;7(1):29-39. doi:10.1513/pats.200909-102JS
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3-12. doi:10.1002/path.2697
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460-473. doi:10.1089/ars.2013.5371
- Schuck, Sebastian. Microautophagy – distinct molecular mechanisms handle cargoes of many sizes. Journal of Cell Science. Published 9 September 2020; 133: jcs246322 doi: 10.1242/jcs.246322
- Ju Huang & Daniel J. Klionsky (2007) Autophagy and Human Disease, Cell Cycle, 6:15, 1837-1849, DOI: 10.4161/cc.6.15.4511
- Nakamura S, Yoshimori T. Autophagy and Longevity. Mol Cells. 2018;41(1):65-72. doi:10.14348/molcells.2018.2333