From chapter 3.1 of “Live Young, Longer” (2016 – English version coming soon).
Now that we have seen how pharmacological and preclinical research works from the previous article, let’s move on to clinical and epidemiological studies and quality standards.
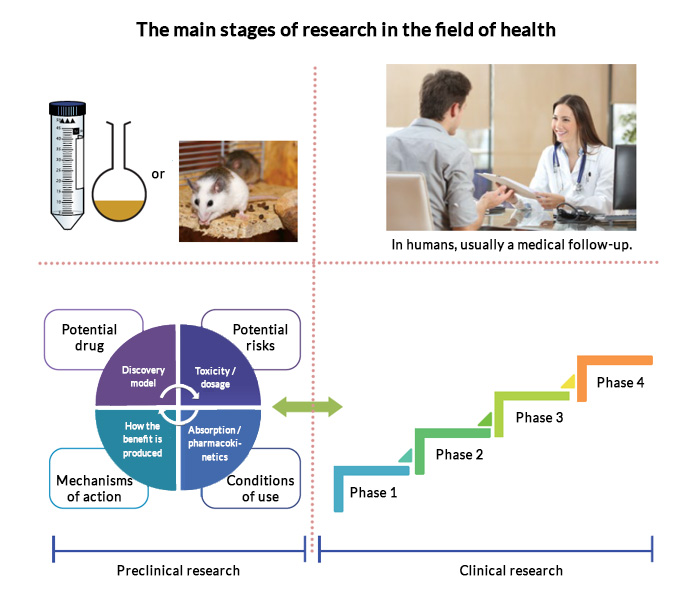
The 4 phases of a clinical study
When the results from the studies mentioned in the previous article are deemed satisfactory and demonstrate efficacy as well as a positive safety profile, the dossier is sent to the responsible government authority to determine the appropriateness of carrying out clinical studies. Note that for each country or group of countries, there is an agency dedicated to the review process for preclinical and clinical records. In Canada, of course, it is Health Canada. If the latter do not agree, the clinical study cannot be carried out. If, on the contrary, they give their consent, the clinical study can begin.
Here are the 4 phases of the clinical study:
Phase 1: This phase evaluates the ADME (absorption, distribution, metabolism, elimination) of the product in healthy individuals. It is also at this level that it is possible to demonstrate the absence of side effects as well as tolerance to the treatment.
Phase 2, also called pilot studies: The therapeutic efficacy and the optimal dose are determined. This step can be accomplished in two parts (one for effectiveness and the other for the optimal dose, respectively).
Phase 3 or pivotal studies: Here we are talking about large studies to demonstrate efficacy on a large number of patients. The total number of patients needed depends on the type of health benefit sought, the magnitude of its potential, and the variation observed in the study population.
Phase 4 or post-market: This phase is referred to as follow-up studies once commercial acceptance has been obtained. These will allow monitoring for the effects of treatment and may be required for issues of potential toxicity. Other possible applications of the treatment can also be identified during this step.
Go back and repeat
In human health research, it is possible to repeat a step or more steps again after obtaining new results this is called iteration feedback. To illustrate this, here is an example: during a clinical study, an observation was noted and requires additional preclinical studies to ensure the absence of toxicological risks or to validate the possibility of another potential application. Therefore, there will be an iteration feedback to revise all of this.
This model of therapeutic application development is a case in point and is strictly controlled. It may not be applicable to natural products or human nutrition.
Natural products and human nutrition
The process is often different when it comes to natural products and human nutrition. When it comes to nutrition, the desired effects for a nutrient may have been known or assumed for quite some time. While to know the health benefits, they often have to be measured in clinical studies. Some natural products base their safety profile on a history of consumption that can span hundreds of years, as is the case with Traditional Chinese Medicine. In general, preclinical work can help to understand the mechanisms of action involved.
A final type of study …
Epidemiological studies are consumer studies generally carried out on a large population. It describes in detail everything that is consumed by large groups of people (often 25,000 to 50,000 people), usually over long periods of time (example 15 to 25 years) in order to make connections by statistical methods between certain elements consumed and certain risks of disease. For example, studies of tens of thousands of people have found that tomato consumption in Italy is linked to reduced cases of prostate cancer. Likewise, we have learned from epidemiological studies that nut consumption is linked to longevity. Several health benefits of the Mediterranean diet come from epidemiological studies.
But what are the quality standards?
Unfortunately, the studies carried out are not all equivalent. There are quality standards that allow studies to be classified according to the scientific rigor that was applied. Particularly for the field of nutrition and natural health products, there are different grades of study. In other words, not all studies are of the same quality and the way they are carried out weighs heavily on the quality of the information they provide.
On the pharmaceutical side, for drug development, all studies must meet the highest standards and it is mandatory to market a product. These studies are also governed by government authorities, up to and including phase 3 clinical studies. Documented evidence is much more demanding in this area. Note that these requirements are also very well framed by good laboratory and clinical practices.
What explains the differences in regulatory requirements between the pharmaceutical sector and that of nutrition or natural health products is the risk/benefit balance. Products with little or no risk to health may benefit from less scientific documentation, especially if the targeted benefit is also lower. We will revisit this in the next article.
Even if the scientific research process requires a lot of time, work and criteria to respect, the benefit justifies the cost, since all this makes it possible to create and market products that meet the desired quality standards. In the case of Vitoli products, we have decided to apply much more demanding production and formulation standards than what is required by Health Canada to market natural health products. This ensures greater quality, a higher level of safety, and above all, greater efficiency of the products. Here is an article that explains how the Vitoli product development approach differentiates itself from others: A Better understanding of the Vitoli quality.
Our next article on scientific research will give you a better understanding of the analysis of research results. It will be a question of efficacy, health benefits and risks.