From chapter 3.1 of the book Vivre jeune plus longtemps (2016) – (Live Younger Longer).
Before a natural product resulting from scientific research is made available on the shelves of a pharmacy, several steps must be completed. To help you understand this process of product development, it is important to understand the reasons behind these different stages that make up traditional scientific research.
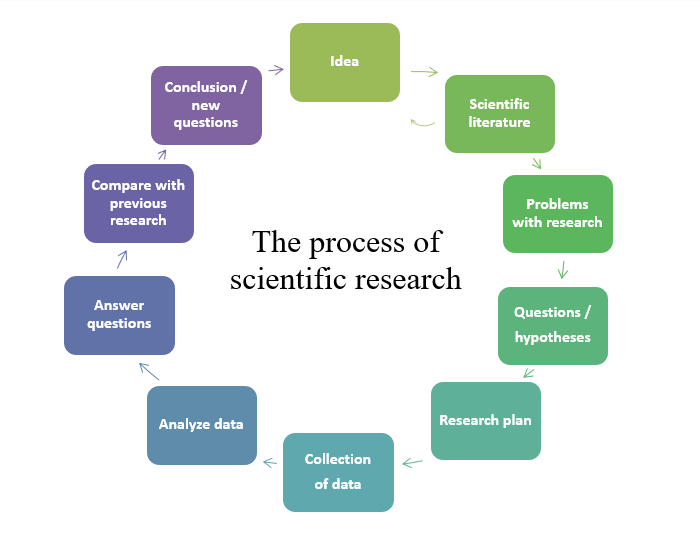
Here are the steps that go into a research process (Please note that these steps are the same in all domains :
- Start with an idea;
- Include the analysis of the literature;
- Develop hypotheses;
- Experiment to affirm or deny the initial hypotheses;
- Analyze the results;
- Draw conclusions and
- Repeat the cycle several times if necessary.
Field of health
When the research process is used in healthcare, two steps are added before clinical studies. Pharmacological and preclinical studies (which include safety studies of a potential drug) should be performed first.
Pharmacological studies
This type of study is basically divided into three parts. Here they are:
- A therapeutic target must be determined for a precise clinical use. It may be a cellular receptor or a metabolic pathway involved in the induction or development of the disease.
- Once this therapeutic target has been identified, various types of molecules or biological products are screened to identify those capable of modulating/blocking the targeted cellular process. Once the class of molecules has been identified, optimization is performed to obtain a molecule with maximum activity. It is also necessary to ensure the quality of the source of these molecules and their presence in sufficient quantity.
- Once the molecule/biological process has been identified, two types of studies are undertaken, often in parallel. The mechanism of action of the molecule/biological process is studied in depth in order to understand the effects and consequences of the interaction of the molecule with the therapeutic target. In parallel, the characteristics of absorption (pharmacokinetics), distribution and metabolism of the molecule are studied. The effectiveness of the molecule is tested in vitro and in vivo using cells and animal models that reproduce the targeted disease.
Preclinical studies
When all the parts related to the pharmacological studies are completed and the effectiveness is proven, it is time to proceed to the preclinical studies. These assess the potential risks of using the molecule. It is here that the maximum tolerance dose of the molecule (risks of toxicity or non-toxicity) will be determined in vitro and in vivo. According to a well-established protocol, this type of study also assesses the safety of the molecule by calculating its effect on the different systems (e.g.: respiratory systems, cardiac and neurological systems, the risks of mutagenesis and carcinogenicity/cancer).
According to tightly supervised procedures, preclinical research can be carried out with the use of typical laboratory and/or cellular equipment. Being essential for analyzing toxicity, mechanisms of action or the comparison between two potential treatments, research on animal models meets specific criteria and requires precise justifications. When the formulation studies are initiated, we can define the profile of absorption, distribution, metabolism and excretion of the molecule (alone or in tablet form).
Here are what the pharmacological and preclinical studies summarize. To learn more about the next steps in scientific research, don’t miss our next articles on the subject, which will explain what clinical studies, epidemiological studies, and research-related quality standards consist of.
This information will help you better understand the justifiable uses of quality natural products by considering the scientific evidence available. Watch out for rhetoric statements about natural health: the truth is, surely not that all supplements are good or all are bad. All those who hold these words, one way or another, are simply demonstrating that:
- Either they do not know enough about the topic to be able to properly assess the usefulness of quality natural health products available on the market,
- Or they have conflicts of interest that skew their statements.
Unfortunately, the quality of products on the market varies widely and few people are really aware of this. These articles on scientific research should help you see more clearly.
References:
- Enna, S.J., Norton, Stata. 2012. Herbal supplements and the brain: understanding their health benefits and hazards. Pearson Education, Inc. Publishing as FT Press. ISBN 978-0-13-282497-2. 273 pp.
- Kelber, O., Nieber, K., and Kraft, K., 2014. Valerian: No Evidence for Clinically Relevant Interactions. Hindawi Publishing Corporation, Evidence-Based Complementary and Alternative Medicine. Volume 2014, Article ID 879396, 8 pages.
- Lakhan, S.E., and Vieira, K.F., 2010. Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutrition Journal 9:42.
- Omar SH. 2010. Oleuropein in olive and its pharmacological effects. Sci Pharm. 78(2):133-54.
- Servili , M., Sordini, B., Esposto, S., Urbani, S., Veneziani, G., Di Maio, I., Selvaggini, R., and Taticchi, A. 2014. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Review. Antioxidants. 3, 1-23.